
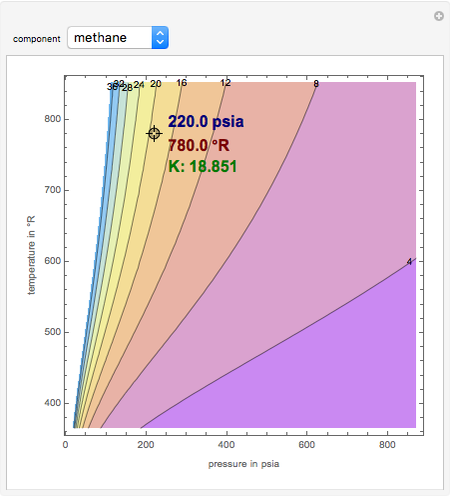
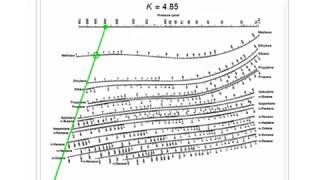
#How to use depriester chart to find temperature software
This file contains additional information such as Exif metadata which may have been added by the digital camera, scanner, or software program used to create or digitize it. Original file (550 × 676 pixels, file size: 67 KB, MIME type: image/jpeg)įile information Structured data Captions English Add a one-line explanation of what this file represents Captions Summary Įnglish: K-Values for systems of light hydrocarbons, high temperature range 40+ Terry Pratchett Audiobook covers ideas | terry pratchett books, terry pratchett, terry pratchett discworld.If the pressure is below the dew point pressure $K_i$ will be larger. This equation directly yields the correspondance of $K$ and $p$. 63 g/ml A flash drum is separating a feed that is 50 wt% n-propanol and 50 wt% isopropanol with F = 100 k mol/h, T = 90^degree C Assume the vapor is an ideal gas to calculate vapor densities. Find the dimensions in metric units required for a vertical flash drum. Find V/F, V, L, liquid mole fraction, vapor mole fraction. The drum operates p_drum = 700 kPa and T_drum = 30^degree C. We wish to flash distill a feed that is 55 mol% ethane and 45 mol% n-pentane. Find the mole fraction of n-butane, Z_B, in the feed. The feed mole fraction of ethane is z_E = 0. Composition – Bubble Point Temperature 82. An auxiliary line is used to assist in these determinations…ĩ.The compositions of the 1 st bubble formed and the last liquid drop can be determined from the enthalpy vs.The bubble point temperature and dew point temperatures can be determined from the enthalpy vs.Converting Weight Fraction to Mole Fraction In GeneralĨ. Although slight, one can begin to see the effect of pressure on the azeotropic point.ħ.Thus, the azeotropic mole fraction is greater at P = 1 Kg/cm 2 than at 1 atm: 0.Converting from wt fraction of the azeotrope to mole fraction:.The Pillars Curriculum for Chemical Engineering It is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behaviour.
It is simply defined as the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure. In thermodynamics, the compressibility factor ( Z), also known as the compression factor or the gas deviation factor, is a correction factor which describes the deviation of a real gas from ideal gas behaviour. This page provides supplementary data to the article properties of water. The term comes from the Greek psuchron (ψυχρόν) meaning "cold" and metron (μέτρον) meaning "means of measurement". Psychrometrics, psychrometry, and hygrometry are names for the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures. The Pillars Curriculum for Chemical EngineeringĪ phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.


 0 kommentar(er)
0 kommentar(er)
